High Impact Tutoring Built By Math Experts
Personalized standards-aligned one-on-one math tutoring for schools and districts
In order to access this I need to be confident with:
Arithmetic Volume Units of measurementConverting measurements
Density formula
Here you will learn about the density formula, including how to calculate density, mass and volume and how to solve problems involving these measures.
Students will first learn about the density formula as part of algebra in 9 th grade.
What is the formula for density?
The formula for density is: \text{Density}=\cfrac{\text{Mass}}{\text{Volume}}.
“Density equals mass divided by volume.”
Density is a compound measure which tells us about the mass of an object in relation to its volume.
For example, if an object has a mass of 500 \, kg and a volume of 2.5 cubic meters then the density would be Density = Mass \div Volume = 500 \div 2.5 = 200 \, kg/m^{3}.
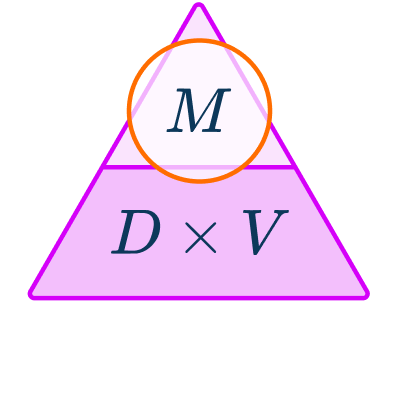
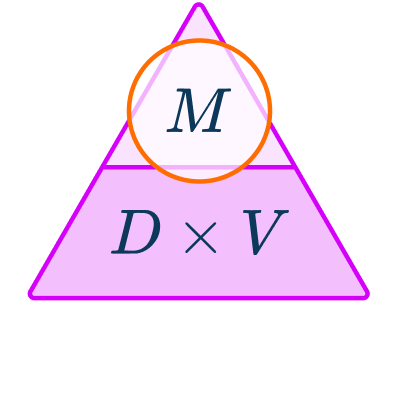
The density formula can be rearranged to calculate mass or volume given the other two measures. An easy way to remember the formula and the different rearrangements is to use this density mass volume triangle.
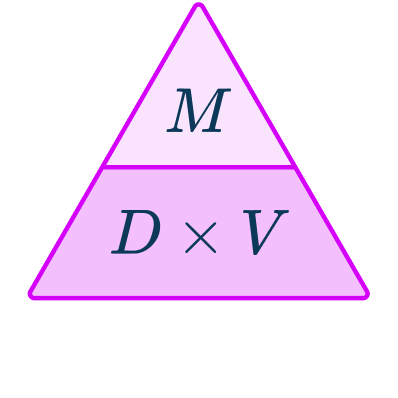
From this triangle you can figure out how to calculate each measure. You can “cover up” what you are trying to find and the formula triangle tells you what calculation to do.
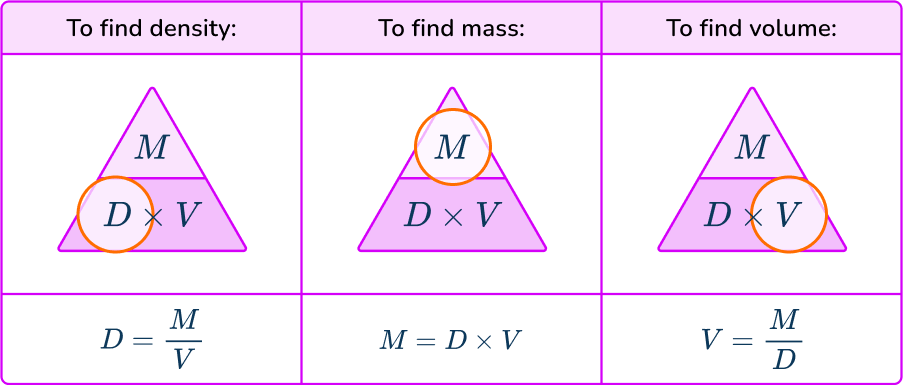
For example, if an object has a density of 750 \, kg/m^{3} and a mass of 225 \, kg then its volume would be Volume = Mass \div Density = 225 \div 750 = 0.3 \, m^{3}.
For example, if an object has a density of 6.8 \, g / cm^3 and a volume of 2.7 \, g/cm^3, then its mass would be Mass = Density \times Volume = 6.8 \times 2.7 = 18.36 \, grams.
What is the formula for density?
![[FREE] Ratio Worksheet (Grade 6 to 7)](https://thirdspacelearning.com/wp-content/uploads/2023/07/Ratio-check-for-understanding-quiz-listing-image-.png)
[FREE] Ratio Worksheet (Grade 6 to 7)
![[FREE] Ratio Worksheet (Grade 6 to 7)](https://thirdspacelearning.com/wp-content/uploads/2023/07/Ratio-check-for-understanding-quiz-listing-image-.png)
Use this quiz to check your grade 6 to 7 students’ understanding of ratios. 10+ questions with answers covering a range of 6th and 8th grade ratios topics to identify areas of strength and support!
DOWNLOAD FREE![[FREE] Ratio Worksheet (Grade 6 to 7)](https://thirdspacelearning.com/wp-content/uploads/2023/07/Ratio-check-for-understanding-quiz-listing-image-.png)
[FREE] Ratio Worksheet (Grade 6 to 7)
![[FREE] Ratio Worksheet (Grade 6 to 7)](https://thirdspacelearning.com/wp-content/uploads/2023/07/Ratio-check-for-understanding-quiz-listing-image-.png)
Use this quiz to check your grade 6 to 7 students’ understanding of ratios. 10+ questions with answers covering a range of 6th and 8th grade ratios topics to identify areas of strength and support!
DOWNLOAD FREEDensity, mass, volume and units of measure
Density is a compound measure and therefore involves two units; a combination of mass in relation to volume.
It is very important to be aware of the units being used when using the density formula to calculate density, mass or volume.
- Examples of units of mass: grams (g), kilograms (kg), milligrams (mg), ounces (oz), pounds (lb).
- Examples of units of volume: cubic centimeters (cm^{3}), cubic meters (m^{3}), liters (l), milliliters (ml), cubic inches (inches^{3}), gallons (gal).
- Examples of units of density: grams per cubic centimeter g/cm^{3}, kilograms per cubic meter kg/m^{3}.
When you use the formula for density, you must check that each measure is in the appropriate unit before completing the calculation. Sometimes you will need to convert a measure into different units. Here are some useful conversions to remember:
Units of Mass:
\begin{aligned}&1 \, g = 1,000 \, mg \\\\ &1 \, kg = 1000 \, g \end{aligned}Units of Volume:
\begin{aligned}&1 \, m^{3}=1000000 \, cm^{3} \\\\ &1 \, liter = 1,000 \, ml \\\\ &1 \, ml=1 \, cm^{3} \\\\ &1 \, liter = 1000 \, cm^{3} \end{aligned}For example, let’s calculate the density of an object that has a mass of 5,300 \, g and a volume of 0.02 cubic meters.
For density, common units used are kg/m^{3} or g/cm^{3}.
The units given in this question are g and m^{3}, so you should convert one of the common units given above.
1,000 \, g=1 \, kgTherefore,
5,300 \, g=5.3 \, kg.Then we can calculate the density in kg/m^{3}, Density = Mass \div Volume = 5.3 \div 0.02 = 265 \, kg/m^{3}.
Note also that sometimes you may need to convert an answer into different units at the end of a calculation.
Density in science
Using the formula for density, you can calculate the density of a variety of things such as the density of water, the density of liquid, and the density of air. The Greek letter \rho (pronounced ‘rho’) is sometimes used to represent density.
In science, there is an international system of units known by the abbreviation SI.
This is the most modern form of the metric system and is most commonly used across the world.
The SI unit for mass is kilograms (kg).
The SI unit for length is meters (m).
The SI unit for volume is cubic meters (m^{3}).
These units would then calculate a density in kilograms per cubic meter (kg/m^{3}).
However, the density unit of grams per cubic centimeters (g/cm^{3}) is often used.
Common Core State Standards
How does this relate to high school math?
- Algebra (HSA.CED.A.4)
Rearrange formulas to highlight a quantity of interest, using the same reasoning as in solving equations. For example, rearrange Ohm’s law V=IR to highlight resistance R.
How to use the density formula
In order to use the density formula to calculate density, mass or volume:
- Write down the measurements known with the units.
- Write down the formula you need to use from the density, mass, volume triangle.
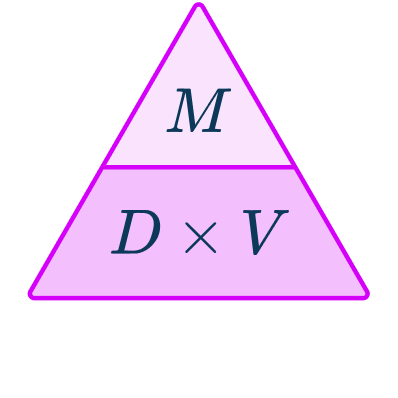
- Check that the units are compatible with each other, converting them if necessary.
- Substitute the values into the selected formula and calculate.
- Record your final answer with units.
Formula for density examples
Example 1: calculating density
A bar is made from aluminum.
It has volume 50 \, cm^{3} and mass 135 \, g.
Calculate the density of the object.
- Write down the measurements known with the units.
2Write down the formula you need to use from the density, mass, volume triangle.
You want to calculate density.
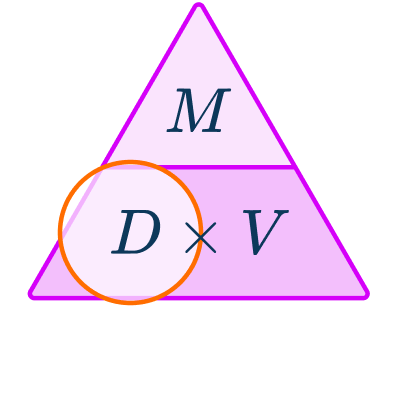
3Check that the units are compatible with each other, converting them if necessary.
The units are g and cm^{3} which are compatible to calculate a density in g/cm^{3}.
4Substitute the values into the selected formula and calculate.
\text{Density}=\cfrac{\text{Mass}}{\text{Volume}}=\cfrac{135}{50}=2.75Record your final answer with units.
2.7 \, g/cm^{3}Example 2: calculating density
A block of ice has a volume of is 576\ cm^{3} and a mass of 530 g.
Calculate the density of the ice.
Give your answer to 3 significant figures.
\begin{aligned}& Density = \; ? \\\\ & Mass = 530 \, g \\\\ & Volume = 576 \, cm^{3} \end{aligned}You want to calculate density.
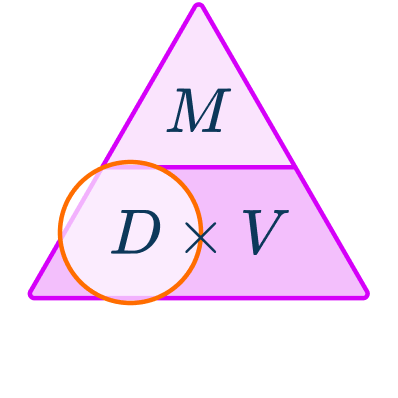
The units are g and cm^{3} which are compatible to calculate a density in g/cm^{3}.
\text{Density}=\cfrac{\text{Mass}}{\text{Volume}}=\cfrac{530}{576}=0.920138….0.920 \, g/cm^{3} (to 3 \, sf )
Example 3: calculating volume
A student weighs a piece of granite and records its mass as 800 \, g. The teacher tells the student that the density of the granite is s 2.5 \, g/cm^{3}.
Using this information, calculate the volume of the piece of granite.
\begin{aligned}& Density = 2.5 \, g/cm^{3} \\\\ & Mass = 800 \, g \\\\ & Volume = \; ? \end{aligned}You want to calculate volume.
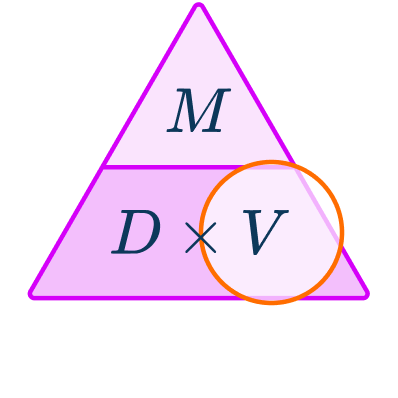
The units are g and g/cm^{3} which are compatible to calculate a volume in cm^{3}.
\text{Volume}=\cfrac{\text{Mass}}{\text{Density}}=\cfrac{800}{2.5}=320 320 \, cm^{3}Example 4: calculating volume with unit conversion
The mass of an object is 2.8 \, kg. The object is made of a substance which has a density of 5.2 \, g/cm^{3}.
Calculate the volume of the object.
Give your answer to 3 significant figures.
\begin{aligned}& Density = 5.2 \, g/cm^{3} \\\\ & Mass = 2.8 \, kg \\\\ & Volume = \; ? \end{aligned}You want to calculate volume.
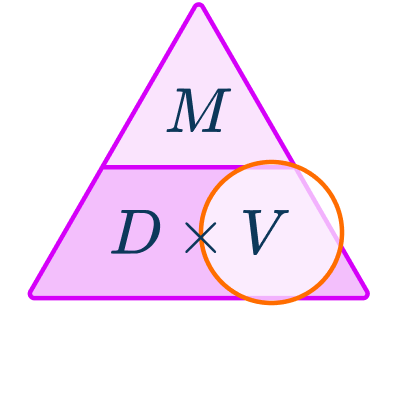
The units are kg and g/cm^{3} which are not compatible to be used in the formula.
You must convert mass from kg into g.
1 \, kg = 1000 \, g
2.8 \, kg=2.8\times 1,000=2,800 \, g
Example 5: calculating mass with unit conversion
Liquid benzene has a density of 0.9 \, g/cm^{3}.
Find the mass of 5 \, liters of liquid benzene.
\begin{aligned}&Density = 0.9 \, g/cm^{3} \\\\ & Mass = \; ? \\\\ & Volume = 5 \, liters \end{aligned}You want to calculate mass.
The units are liters and g/cm^{3} which are not compatible to be used in the formula.
Convert the volume from liters into cm^{3}.
1 \, liter = 1,000 \, cm^3 so,
5 \, liters = 5\times 1,000=5,000 \, cm^3
You can now use the formula and calculate the mass in grams.
Example 6: calculating mass with unit conversion
Iron has a density of 7.8 \, g/cm^{3}.
Find the mass of 620 \, cm^{3} of iron.
Give your answer in kilograms to 2 significant figures.
\begin{aligned}& Density = 7.8 \, g/cm^{3} \\\\ & Mass = \; ? \\\\ & Volume = 620 \, cm^{3} \end{aligned}You want to calculate mass.
The units are cm^{3} and g/cm^{3} which are compatible to calculate the mass in grams.
\text{Mass}=\text{Density}\times \text{Volume}=7.8\times 620=4,836 \begin{aligned}& 4,836 \, g \\\\ & 1 \, kg = 1,000 \, g \\\\ & 4,836 \div 1,000 = 4.836 \\\\ & 4.8 \, kg \end{aligned}Teaching tips for the density formula
- Explain how the unit of volume can be a capacity unit, like liters, but it can also be a calculated unit like cm^3.
- Always bring attention to the density units and consider how they are interpreted. For example, from a density of 7,800 \mathrm{~kg} / \mathrm{m}^3 to 7.8 \mathrm{~g} / \mathrm{cm}^3 it appears that there is a decrease. However, when you consider the units it becomes clear that the second value is not less dense, but equivalent.
- Instead of just using worksheets, let students experience density hands on. This can be as simple as pouring immiscible liquids into the same container, observing what happens and making conjectures about the density of substances in the container.
- Use the topic of density to make connections to other disciplines when possible; such as Earth science, Biology, Statistics, English, etc.
- Allow struggling students to use a density calculator to explore the relationship until they are comfortable completing the calculations on their own.
Easy mistakes to make
- Thinking that density cannot be calculated because the volume is missing
When you are asked to calculate density you need to know what the mass and volume are. In some cases you will simply be given the volume but in other cases you may have to calculate the volume for yourself. Remember the volume of a rectangular prism is the product of its three dimensions.
For example,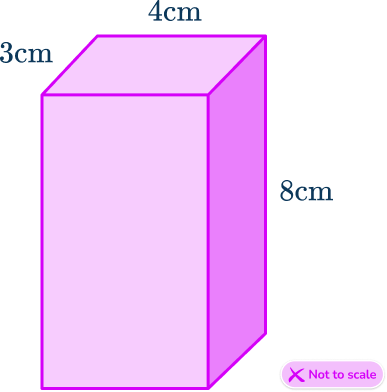
\text{Volume}=3\times 4\times 8 = 96 \, cm^3
See also: Volume formula
- Using incompatible units in a calculation
When using the formula for density you must ensure that the units of the measures are compatible. Density is usually measured in g/cm^{3} or kg/m^{3}. This table shows the compatible units.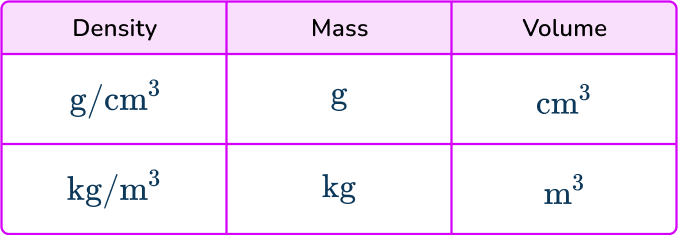
So, for example, if you are given a mass in grams but a volume in m^{3} then these are not compatible and you must convert one of the measures.
- Doing the correct calculation but writing your final answer incorrectly
Always think about the units of your final answer. Read the question carefully and check to see if any specific units are required. In some cases you may have to convert your answer into different units after a calculation.
Also check to see if the question requires your answer to be to a certain degree of accuracy such as one decimal place, or 2 significant figures.
Practice formula for density questions
1. Find the density of the material with a mass of 900 \, kg and a volume of 300\ m^{3}.




2. Find the density of an object which has a total volume of 690 \, cm^3 and a mass of 950 \, g. Give your answer to 3 significant figures.




3. Find the volume of an object which has a mass of 600 \, g and a density of 12 \, g/cm^3.




4. Find the volume of an object which has a density of 10.4 \, kg/m^3 and a mass of 230 \, kg. Give your answer to 3 significant figures.




5. The density of a substance is 8 \, g/cm^3. If there is 480 \, g/cm^3 of this substance, what is the mass in kg?




But the question asks for the answer in kg.
3,840 \, g= 3.84 \, kg
6. The density of this cube is 11.2 \, g/cm^3. Find the mass.
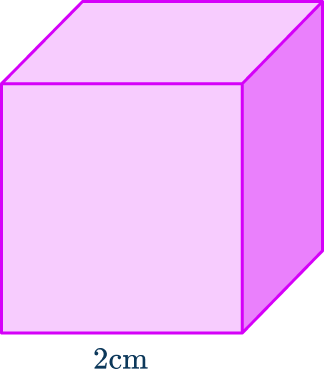




First we need the volume of the cube.
\text{Volume}=2\times 2\times 2=8 \, cm^3
Then we can find the mass by multiplying the density by the volume.
\text{Mass}=\text{Density}\times \text{Volume}=11.2\times 8=89.6 \ , g
Density formula FAQs
Buoyancy is the upward force that impacts whether or not an object or substance will float or sink when in a liquid. Objects/substances that are less dense than the liquid they are in will float. Objects/substances that are more dense than the liquid they are in will sink.
The formula for density is \rho=\cfrac{m}{V}, where \rho is density, m is mass and V is volume.
The next lessons are
Still stuck?
At Third Space Learning, we specialize in helping teachers and school leaders to provide personalized math support for more of their students through high-quality, online one-on-one math tutoring delivered by subject experts.
Each week, our tutors support thousands of students who are at risk of not meeting their grade-level expectations, and help accelerate their progress and boost their confidence.
Find out how we can help your students achieve success with our math tutoring programs.
[FREE] Common Core Practice Tests (3rd to 8th Grade)
Prepare for math tests in your state with these 3rd Grade to 8th Grade practice assessments for Common Core and state equivalents.
Get your 6 multiple choice practice tests with detailed answers to support test prep, created by US math teachers for US math teachers!